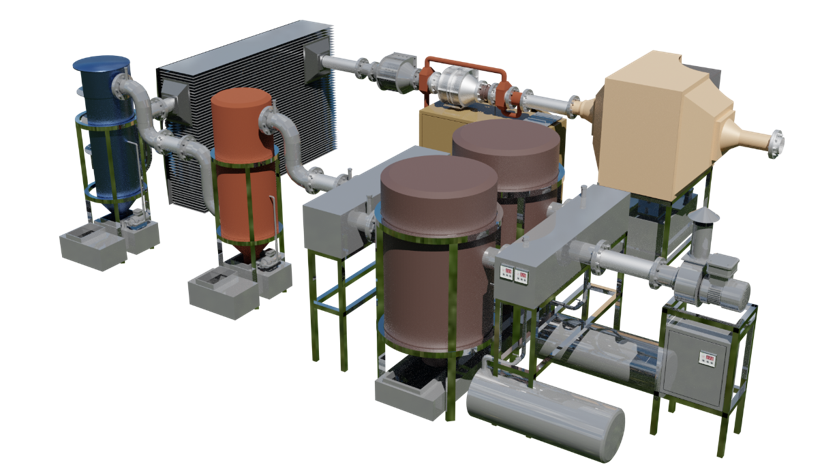
This stage is designed for adsorption absorption of CO 2 using a solid sorbent.
The essence of the method lies in the fact that in practice, gas, previously purified from dust and harmful oxides, is passed through an adsorbent layer: the latter is most often zeolites of various grades ( NaA , NaX , CaA ), characterized by certain pore sizes and, as a result, , molecular sieve properties that promote selective absorption of CO 2 .
Possible potential absorbers include materials containing soda, which reacts with CO 2 to form bicarbonate and its further decomposition during desorption according to the scheme:
Na 2 CO 3 + CO 2 + H 2 O \u003d 2NaHCO 3 2NaHCO 3 \u003d Na 2 CO 3 + CO 2 + H 2 O.
The adsorption process proceeds at room temperatures (5–35°C) and pressures close to atmospheric. Adsorption proceeds until the sorbent is saturated, after which the adsorption cycle is completed and the gas flow is redirected to another adsorber, and in the first one, the stage of regeneration with live steam occurs at temperatures of 100–130°C. The stages of adsorption and desorption proceed alternately with automated direction of gas and para- desorbent flows . After desorption, the steam with carbon dioxide is sent to a refrigerator, where CO 2 is separated from the condensing water. This method is more technological and economical.
The adsorption method of CO 2 absorption proposed in this project has a number of advantages over those currently used in practice. One of the main advantages is the use of solid waste from thermal power plants as the main part of the sorbent.
In this case, depending on the external conditions, various modifying additives and sorbent preparation schemes can be used.
This approach will allow creating flexible systems that can be easily adapted to the specific operating conditions of thermal units.
Since CHP ash is characterized by a small specific surface area, it is advisable to introduce binder materials of various nature into its composition, which will allow both the formation of a sorbent and the achievement of the required characteristics of its porous structure, which are necessary for effective absorption of CO 2 . In particular, preliminary experiments have shown the fundamental possibility of forming granules of a soda-containing adsorbent based on CHP waste with a satisfactory efficiency of CO 2 absorption.

